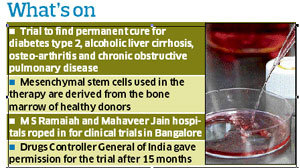
 歷經15個月的審查,印度的藥物管控衛生機構(Drug Controller General of India: DCGI)日前一次同意四個慢性疾病的間葉幹細胞臨床試驗,包括慢性阻塞性肺病(chronic obstructive pulmonary disease: COPD),第二型糖尿病(diabetes type 2),酒精性肝纖維化(alcoholic liver cirrhosis)和關節炎(osteoarthritis),於四月在印度的五個城市進行,完整試驗將為期三年,預計2014會有結果報告出爐.
Stempeutics Research 幹細胞公司負責這次的臨床試驗,於去年曾向國家藥物管理局申請間葉幹細胞對心臟病的臨床試驗,一年多來已完成安全性的評估.因此這次針對慢性疾病的臨床試驗直接進入第二期的階段
第二期的臨床試驗是針對小規模病患進行治療性的評估,預計在慢性阻塞性病患有30人;糖尿病病患30人;肝硬化病患60人;關節炎病患45人,一共近200人進行這次的臨床試驗.治療方式是抽取健康捐贈者的骨髓,分離出間葉幹細胞並進行培養,增殖後的間葉幹細胞再打入病患受損的位置,直接進行修補
間葉幹細胞經實驗證實,可分化出三個胚層的細胞,例如肺臟,胰島,肝臟,骨骼,心肌等細胞,因此打入受損位置可以因分化而再生新的組織
間葉幹細胞不只分布於骨髓,在剛出生嬰兒的臍帶組織中,也富含間葉幹細胞.這些間葉幹細胞經萃取培養後能進行儲存,以提供未來的疾病治療
看似保守的印度政府已同意並進行間葉幹細胞的臨床試驗,相信我國衛生機關在不久之後,也能開放間葉幹細胞的人體臨床試驗,讓我國醫療能與國際接軌,並越來越進步
First nod for stem cell clinical trial
|
|
New Delhi, Feb. 15: India’s first set of government-approved clinical trials of stem cells on patients with chronic obstructive lung disease, diabetes, liver cirrhosis and osteoarthritis are likely to begin in five cities in April this year. A Bangalore-based company, Stempeutics Research, has received approval from the country’s drug regulatory agency to evaluate the efficacy of its stem cells on these four incurable diseases after safety assessments over the past year on patients with cardiovascular disease. The efficacy — Phase II —trials are likely to begin on small groups of volunteer patients offered the experimental treatment in collaborating hospitals in Bangalore, Kochi, Delhi, Mangalore and Manipal, a senior Stempeutics official said. Each volunteer patient will receive a dose of mesenchymal stem cells derived from the bone marrow of healthy persons. The stem cells, coaxed to proliferate in a broth of laboratory biochemicals, will be injected at the site of illness — the pancreas, the liver, the lungs, or the bone — where they are expected to stimulate resident stem cells and regenerate the damaged or lost tissue. While private and even government hospitals have in the past offered stem cell therapy to patients with intractable conditions, the proposal by Stempeutics is the first with formal approval from regulators for chronic obstructive pulmonary disease (COPD), diabetes, liver cirrhosis and osteoarthritis. The Phase II trials will involve testing the mesenchymal stem cells on 30 patients with COPD, 30 with diabetes, 45 with osteoarthritis, and 60 with liver cirrhosis, distributed across the five cities, Stempeutics chief executive B.N. Manohar told The Telegraph. The proposal for the trials was screened by a panel of experts of the Indian Council of Medical Research. The evaluation of the results is expected to take several months, and Manohar cautions that a commercial therapy may emerge only by early 2014. Stem cells are progenitor cells that have the capacity to turn into other cell types, such as liver cells, insulin-secreting pancreatic cells, bone cells, lung cells or even cells of the heart muscle. Stempeutics has already conducted safety tests of mesenchymal stem cells on patients who have suffered damage to their heart muscles from heart attacks. These trials, which began more than a year ago, have shown that the stem cell injections are safe. |


 留言列表
留言列表